Jose Antonio Carrero, Gorka Arana and Juan Manuel Madariaga
Department of Analytical Chemistry, University of the Basque Country, (UPV/EHU), PO Box 644, 48080 Bilbao, Spain. E-mail: [email protected]
Introduction
Road transport emissions have become one of the most serious environmental problems in many cities and their contribution to the global emission of atmospheric pollutants is increasing every year.1 The dispersion of these contaminants is determined by weather conditions, and metals may be deposited in surrounding areas such as roadside soil2,3 and building facades.4 In fact, there are many studies where high levels of toxic metals (Pb, Ba, Zn, Cd, Sb and Cu among others) have been reported as a consequence of traffic emissions.5,6,7,8 Well-known traffic-related metal emission sources are: brake linings, tyres, exhaust fumes and galvanised traffic signals.9,10,11 Metal pollution is confined to a narrow area alongside the road, but not limited to the soil surface.3,6 What is less certain is the state in which the metals are found in the soil. They can be more or less mobile in the soil, in relation to their geochemical form, which affects their solubility and, thus, their bioavailability.12 The total concentration of pollutants is not very representative of the potential toxicity and it is essential to know the chemical form of the metals.13 Contaminated roadside soils act as secondary pollution sources and may pose a risk to ecosystems and human health, as well as to the built heritage if they are transferred to other reservoirs. For instance, these pollutants can be transported to the aerial parts of the vegetation, bioaccumulating in them.14,15 Therefore, metal speciation by molecular spectroscopy techniques is an important task in order to evaluate metal mobility and potential bioavailability of hazardous compounds. The use of Raman spectroscopy provides structural and molecular information about the compounds originated by traffic emissions and gives an idea of the potential risk of the pollutants in roadside soil.16 The study presented here was carried out in order to assess the synergic impacts of the guardrails and traffic pollutants on urban roadside soils and plants and to understand the mechanism of metal release. Soils, plants and guardrails from a highly traffic-dense road in the urban area of Bilbao (Spain) were selected to carry out the investigation.
Instrumentation
Raman analysis was performed using three different devices. A first screening of the guardrail was done using a hand-held innoRam Raman spectrometer (B&WTEK, Inc., Newark, USA) equipped with a 785 nm excitation laser. Then, spots of 10 µm or greater were measured for guardrail and soil samples, using a portable Renishaw RA100 (Renishaw, UK) spectrometer, equipped with a 785 nm diode laser and a 20× objective. Other analyses were performed using an inVia Raman microspectrometer (Renishaw, UK) coupled to a Leica DM LM microscope (Leica Microsystems, UK). This system was used with a laser of 514 nm (ion–argon laser). Elemental measurements directly on the surface of the guardrail and on the soil samples were carried out using a portable ARTAX µ-ED-XRF (energy dispersive X-ray fluorescence; Bruker AG, Germany) equipped with an X-ray tube with a molybdenum anode working at 50 kV and 0.6 mA.
Analytical approach
The analytical procedure consisted of first undertaking a µ-ED-XRF analysis in order to determine the elemental composition of the samples and the distribution of the elements, then followed by a Raman spectroscopic analysis to obtain their molecular composition. The elemental chemical information was used to help in the interpretation of the molecular data.
The calibration of the Raman spectrometer was carried out daily using the 520 cm–1 silicon band. The measurement conditions vary depending on the gathered signal-to-noise ratio and the obtained fluorescence, but exposure times from 5 s to 30 s and 1–10 accumulations at 1% or 10% power were used in most cases. Spectra were recorded with the WIRE (Renishaw, UK) software between 2200 cm–1 and 200 cm–1 (spectral resolution of 1 cm–1 and cut-off of the system around 200 cm–1) and processed with Omnic 7.2 (Nicolet, Madison, WI, USA) software. The interpretation of the results was performed by comparing the obtained spectra with standard spectra contained in our own home-made Raman database17 and those collected in the RRUFF database.18
The clay matrix of the soil gives a high fluorescence signal, which can saturate the Raman signal and make the interpretation of the spectra difficult. Short acquisition times at low intensity power were used for spectra acquisition in order to avoid saturation but, even so, high fluorescence was obtained and the resulting spectra were too noisy. Thus, a pre-treatment of the spectra was carried out prior to identification. In most cases, correction of the baseline and smoothing was used to a greater or lesser extent.
Results and discussion
High Zn concentration in soil samples just under the guardrail was detected in one of our previous works.8 Therefore, in order to explain this anomalous Zn concentration, Raman spectroscopy analysis were carried out on the guardrail as a potential source of Zn impacting the soil under it. Interpretation of the Raman spectra can sometimes be very difficult without first having an idea of the elemental composition and concentration range of the different elements present in the sample analysed. Therefore, a first screening by means of XRF helps in the interpretation of the obtained Raman spectra. As can be seen in the XRF spectrum in Figure 1(a), some elements related to the traffic (Ba, Pb, Ca, Sr) were detected in the surface of the guardrail, as well as those originally from the guardrail (Zn and Fe).16 The Raman spectra in Figure 1(b) of the old and deteriorated guardrail shows the presence of a basic carbonate of zinc, namely hydrozincite [Zn5(CO3)2(OH)6]. This means that the Zn layer of the galvanised steel (supposedly a layer of ZnO is covering the Zn plate) has degraded due to the strong corroding conditions to which these traffic controls are exposed, in an environment where thousands of vehicles pass and emit exhaust gases (COx, NOx and SOx). In this particular case, the hydrozincite can be formed in situ by a reaction between the atmospheric CO2(g) with the ZnO(s) at high humidity levels:
5 ZnO(s) + 2 CO2(g) + 3 H2O ↔ Zn5(CO3)2(OH)6(s)
This Zn then occurs in the soil beneath, where it can undergo new reactions and form other more soluble species. In fact, Zn(NO3)2 was detected in soil, as can be seen in the Raman spectrum in Figure 2 left. The formation of this new compound can be explained by the wet acid attack of the oxidised NOx (in fact nitric acid aerosol) on the hydrozincite:
Zn5(CO3)2(OH)6(s) + 10 HNO3 ↔ 5 Zn2+ + 10 NO3– + 2 CO2(g) + 8 H2O


When the rain starts, the dissolved zinc and nitrate ions drop from the guardrail to the soil. Both ions can then be recombined as Zn(NO3)2 when water evaporates from the soil.
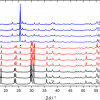
The soil matrix is very complex and obtaining a good Raman spectrum of it is a difficult task. The number of different compounds that could be present in the soil is very broad. Once again, the previous analysis of the samples by means of XRF analysis can be very helpful in the interpretation of the recorded Raman spectra. Furthermore, the clay matrix, typical of soil samples, gives a high background Raman signal and the less intense bands (weak) are covered by this fluorescence emission. In most cases, only the principal bands (very strong) can be clearly observed in the spectra. It is common to obtain spectra of some of the major components of the soil, such as quartz (SiO2), calcite (CaCO3), silicates and different crystalline forms of titanium and iron oxides. Figure 2 shows the Raman spectra of TiO2 (anatase, rutile and brookite). Spectra of albite (NaAlSi3O8), goethite (FeOOH) and hematite (Fe2O3) are not shown. The spectra containing anatase and litharge bands in Figure 2 have some other not fully attributed bands. They seem to correspond to other oxides and, possibly, to an organic complex form of Cu, because some of the peaks match well with the Raman spectra of the Cu–O bands.
In this work, BaCO3 was detected, both on the guardrail and in soil samples (Figure 2). Ba is used as an additive in tyre rubber and emitted to the atmosphere as a consequence of traffic emissions. The surface of the guardrail serves as a support onto which BaO particles deposit and react with CO2 to form the carbonate of barium. Then, the new generated particles of BaCO3 can be removed by the wind and fall down into the soil, without the need to dissolve by a CO2 acid attack and reprecipitate again as barium carbonate in the soil matrix.
Leaded gasoline was banned in Spain in 2001, but high levels of Pb concentration still remain in roadside soils today. Raman analysis of the soil reveals the presence of litharge (PbO), see Figure 2. This form of lead is not very mobilisable and accumulates in top soil, which explains the high Pb concentration in roadside soil ten years after the phase-out of its use as an additive in gasoline.
The study was completed by analysing vegetation samples found at the roadside. Plant samples were not washed before their analysis. The laser was focused on different particles on the leaves which came from the atmospheric deposition in order to try to identify traffic emitted particles. Only some of the major components of the soil (quartz and calcium carbonate) were found, showing, in this case, that the source is the deposition of the soil turned over by the wind and passage of vehicles. Figure 3 shows the Raman spectra of some particles on plant samples corresponding to calcite and quartz together with amorphous carbon. This appears frequently when analysing organic samples due to the photo-thermo decomposition of leaves.

Concluding remarks
In this study, the combination of Raman spectroscopy and µ-ED-XRF proved to be very efficient. With the combination of these complementary analytical techniques, it has been possible to analyse several roadside soils and plants, as well as a guardrail. The task is not trivial because the number of compounds present in these samples is large. The advantages of Raman spectroscopy to analyse these samples are achieved through the possibility of focusing on individual grains, thereby obtaining the spectrum of each grain that comes from traffic-emitted particles. The results obtained highlight the importance of the use of such analytical tools to detect the molecular species found on the guardrail, soil and plants, which is fundamental in order to be able to explain the reaction path that made them. Further analysis by means of coupled scanning electron microscopy–Raman spectroscopy would facilitate the identification of more traffic particles.
Acknowledgements
This work has been financially supported by the Spanish Ministry of Science and Innovation through the IMDICOGU project (ref.: BIA2008-06592). J.A. Carrero is grateful to the University of the Basque Country (UPV-EHU) for his post-doctoral contract. The authors are grateful for the technical and human support provided by the Raman-LASPEA Laboratory of the SGIker (UPV/EHU, MICINN, GV/EJ, ERDF and ESF).
References
- R.B. Wei and L. Yang, “A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China”, Microchem. J. 94, 99 (2010). doi: 10.1016/j.microc.2009.09.014
- F. Amato, M. Pandolfi, T. Moreno, M. Furger, J. Pey, A. Alastuey, N. Bukowiecki, A.S.H. Prevot, U. Baltensperger and X. Querol, “Sources and variability of inhalable road dust particles in three European cities”, Atmos. Environ. 45, 6777 (2011). doi: 10.1016/j.atmosenv.2011.06.003
- B. Viard, F. Pihan, S. Promeyrat and J.-C. Pihan, “Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails”, Chemosphere 55, 1349 (2004). doi: 10.1016/j.chemosphere.2004.01.003
- N. Prieto-Taboada, M. Maguregui, I. Martinez-Arkarazo, M. Olazabal, G. Arana and J. Madariaga, “Spectroscopic evaluation of the environmental impact on black crusted modern mortars in urban–industrial areas”, Anal. Bioanal. Chem. 399, 2949 (2011). doi: 10.1007/s00216-010-4324-1
- B. Skrbic, S. Milovac and M. Matavulj, “Multielement profiles of soil, road dust, tree bark and wood-rotten fungi collected at various distances from high-frequency road in urban area, Ecological Indicators 13, 168 (2011). doi: 10.1016/j.ecolind.2011.05.023
- X. Chen, X. Xia, Y. Zhao and P. Zhang, “Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China”, J. Hazard. Mater. 181, 640 (2010). doi: 10.1016/j.jhazmat.2010.05.060
- J.A. Carrero, I. Arrizabalaga, N. Goienaga, G. Arana and J.M. Madariaga, in Urban Environment, Alliance for Global Sustainability Bookseries, Ed by S. Rauch and G.M. Morrison. Springer, Dordrecht, The Netherlands, p. 383 (2012).
- J.A. Carrero, N. Goienaga, O. Barrutia, U. Artetxe, G. Arana, A. Hernandez, J.M. Becerril and J.M. Madariaga, in Highway and Urban Environment, Ed by S. Rauch, G.M. Morrison and A. Monzon. Springer, Dordrecht, The Netherlands, p. 329 (2010).
- D.S.T. Hjortenkrans, B.G. Bergback and A.V. Haggerud, “Metal emissions from brake linings and tires: case studies of Stockholm, Sweden 1995/1998 and 2005”, Environ. Sci. Technol. 41, 5224 (2007). doi: 10.1021/es070198o
- K. Adachi and Y. Tainosho, “Characterization of heavy metal particles embedded in tire dust”, Environ. Int. 30, 1009 (2004). doi: 10.1016/j.envint.2004.04.004
- T.B. Councell, K.U. Duckenfield, E.R. Landa and E. Callender, “Tire-wear particles as a source of zinc to the environment”, Environ. Sci. Technol. 38, 4206 (2004). doi: 10.1021/es034631f
- D.C. Adriano, Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals. Springer, New York, USA (2001).
- A. Kabata-Pendias and H. Pendias, Trace Elements in Soils and Plants. CRC Press, New York, USA (2001).
- O. Barrutia, J.A. Carrero, A. Hernandez, U. Artetxe, G. Arana, J.I. Garcia-Plazaola, J.M. Madariaga and J.M. Becerrill, R.-U. Helmholtz Centre Environmental, in Consoil 2008: Theme D—Risks & Impacts, Vols 1 and 2, Ed Helmholtz Centre Environmental Research-Ufz, Leipzig, p. 391 (2008).
- M. Rucandio, M. Petit-Domínguez, C. Fidalgo-Hijano and R. García-Giménez, “Biomonitoring of chemical elements in an urban environment using arboreal and bush plant species”, Environ. Sci. Pollut. Res. 18, 51 (2011). doi: 10.1007/s11356-010-0350-y
- J.A. Carrero, N. Goienaga, M. Olivares, I. Martinez-Arkarazo, G. Arana and J.M. Madariaga, “Raman spectroscopy assisted with XRF and chemical simulation to assess the synergic impacts of guardrails and traffic pollutants on urban soils”, J. Raman Spectrosc. in press (2012). doi: 10.1002/jrs.4089.
- K. Castro, M. Perez-Alonso, M.D. Rodriguez-Laso, L.A. Fernandez and J.M. Madariaga, “On-line FT-Raman and dispersive Raman spectra database of artists’ materials (e-VISART database)”, Anal. Bioanal. Chem. 382, 248 (2005). doi: 10.1007/s00216-005-3072-0
- Integrated database of Raman spectra, X-ray Diffraction and Chemistry Data for Minerals, www.rruff.info, accessed in October 2011