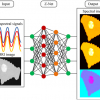
A small group of researchers, including Dennis Kurzbach from the Faculty of Chemistry of the University of Vienna, have described an advanced NMR method to monitor fast and complicated biomolecular events such as protein folding. For example, protein folding was long considered as one of the great mysteries of modern research. This crucial process during which amino acid chains adopt a 3D structure and functionality, takes place within milliseconds. Being this fast, protein folding events could often not be characterised by NMR spectroscopy; the standard method for studying molecular structures. Employing hyperpolarised water, researchers have now developed a method that dramatically enhances the signals of the proteins, nucleic acids and other biomolecules. This renders monitoring of processes such as protein folding possible.
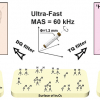
With hyperpolarisation methods, more precisely dissolution dynamic nuclear polarisation (D-DNP), a signal enhancement of over 10,000-fold is possible. “The hyperpolarised water acts as a booster for the NMR signals of a protein during the measurement. The hydrogen nuclei of the hyperpolarised water are exchanged with those of the proteins, thus transferring the signal strength to the latter”, says Dennis Kurzbach.
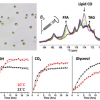
With the new method, the researchers can record an NMR spectrum every 100 ms and use it to track the 3-D coordinates of individual amino acids and how they change over time. “This allows us to monitor processes that occur in milliseconds and distinguish individual atoms”, says Dennis Kurzbach.
In their study the authors describe their technique in detail, from hyperpolarisation to the transfer of the hyperpolarised water to the NMR spectrometer, to the mixing of the hyperpolarised water with the sample solution, and the NMR measurement. In addition, they present six examples for method application, including the observation of protein folding or even the interactions of RNA (nucleic acids) and RNA-binding proteins as the basis for gene expressions in the cell. According to the scientists, the new method can be used for specific studies of RNA, DNA and polypeptides, especially when signal enhancement reaches the “magic” number of 1000-fold.