Surface-enhanced Raman scattering (SERS) has attracted attention in biotechnology due to its high sensitivity to localised surface plasmon resonance of nanostructured metals. Trace detection of bio-molecules with large molecular weight remains challenging because treating SERS substrate using coupling or cross-linking agents is required. A new approach applied liquid-interface assisted SERS (LI-SERS) to realise label-free trace detection of bio-molecules. The results suggest it is promising for early-stage diagnosis of virus infection and Alzheimer’s Disease.
The SERS technique may be used in the bio-medical field for disease diagnosis at an early stage and also in tumour therapy. Although the enhancement factor of SERS typically ranges from 106 to 108 due to the use of novel SERS substrates and methods, single-molecule detection by label-free SERS is impracticable because of SERS-blinking, the origin of this phenomenon is due to the escape of analyte molecules from hotspots. Moreover, bio-molecules, including deoxyribonucleic acid (DNA) and proteins, are difficult to detect directly by SERS. Additional treatments with a SERS substrate are needed to bind the bio-molecules.
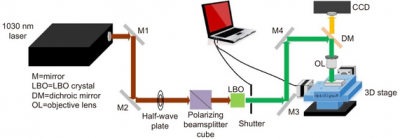
LI-SERS can achieve a SERS enhancement factor greater than 1014, much higher than the regular SERS method. The microfluidic SERS chip featured an Ag–Cu SERS substrate integrated into an embedded glass microchannel. Hybrid femtosecond (fs) laser processing is used to create the glass microchannel.
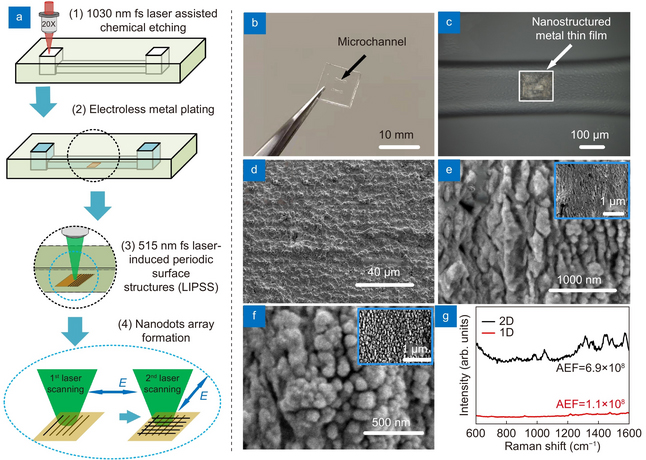
Figure 2. (a) Schematic of the fabrication; (b) photograph of microfluidic SERS chip; (c) optical microscope image showing the SERS substrate. SEM images of (d) original metal film, (e) ripples generated by first laser scanning and (f) nanodots generated by second laser scanning (insert: low magnification of SEM image). (g) Raman spectra of 10–9 M Rhodamine 6G (R6G) on 2-D (black) and 1-D (red) nanostructured SERS substrates. Credit: OEA
The hybrid fs laser processing enables the creation of more complicated 3-D structures with enhanced functionalities for biochips, sensors and microelectronic devices. When the interface between the analyte solution and air on the SERS substrate in the microfluidic channel was irradiated by the Raman excitation laser, the LI-SERS intensity was increased by six orders of magnitude compared with regular SERS. The mechanism of LI-SERS was attributed to the synergetic effect of the Marangoni flow induced by laser irradiation and optical trapping. That laser irradiation would direct the analyte molecules to the hot spots where the collected molecules are trapped by optical force. Consequently, the analyte molecules were immobilised on the SERS substrate with the achievement of strong Raman scattering.
The researchers demonstrated that the LI-SERS method is applicable for more practical use. It is specifically useful for trace detection of label-free bio-molecules with large molecular masses, including DNA bases, DNA sequences and β-Amyloid (Aβ). Owing to the ultrahigh sensitivity and self-immobilisation of LI-SERS, discrimination of DNA bases and DNA sequences with a detection limit of 1 fM was obtained without requiring additional treatments featuring coupling or cross-linking agents. Moreover, the LI-SERS technique can detect label-free Aβ, a biomarker of Alzheimer’s Disease, at levels below 1 pM, and with a linear correlation between the Raman signal and the Aβ concentration in the range 1 nM to 1 pM being achieved. The label-free bio-sensing capability of LI-SERS offers great potential for the early-stage diagnosis of diseases in clinics.