S. Mattssona and J. Börjessonb
aDepartment of Radiation Physics, Lund University, Malmö University Hospital, SE-205 02, Malmö, Sweden
bDepartment of Diagnostic Radiology, County Hospital, SE-310 85 Halmstad, Sweden
Introduction
X-ray fluorescence (XRF) is the emission of characteristic secondary (or fluorescent) x-rays from a material that is excited by bombardment of high-energy x-rays or gamma rays. The process in which a photon is absorbed by an atom by transferring all of its energy to an electron is called the photoelectric effect. Secondary to this process, provided that the photon has a sufficient energy, an electron is ejected primarily from an inner shell, creating a vacancy. This is an unstable condition for the atom and when it returns to its stable condition, an electron from an outer shell is transferred to one of the inner shells. In this process, a characteristic x-ray, whose energy matches the difference between the two binding energies of the corresponding shells, may be created. Because each element has a unique set of energy levels, each element produces a unique set of x-ray energies, allowing us to non-destructively measure the elemental composition of a sample.
The principle of XRF has been known since 1913, when Henry G.J. Moseley (University of Manchester, UK) found the relation between the atomic number of an element (Z) and the wavelength, or energy, of the characteristic x-rays emitted. Moseley measured and plotted the x-ray frequencies for about 40 elements in the periodic table. He showed that the Kα x-rays followed a straight line if Z versus the square root of frequency was plotted, i.e. the Moseley plot. For measurements, Moseley used an x-ray spectrograph with a flat crystal and a photo plate. It had shortly before been constructed by W.H. and W.L. Bragg.
In the early years, the XRF technique was used for element identification, but was not very useful for quantitative analysis and it was not until the development and broader introduction of solid-state detectors for γ- and x-ray spectrometry that the technique started to develop. The first applications were for quantitative and specific analysis in metallurgy, the mining industry and metal prospecting. Later it was also used for art identification purposes and for research in geochemistry and archaeology. Similar types of equipment were occasionally used for measurement on human samples, especially to solve forensic problems.
Later on, interest for measurements on human samples of blood, urine, hair, nails, skin, biopsies etc. has grown steadily, benefiting from the intensive development of new x-ray technologies, e.g. µ-XRF, total reflection XRF (TXRF), the advantages of polarised x-ray beams and synchrotron radiation Röntgen-fluorescence analysis (SR-RFA). In parallel, there has been a dramatic development of alternative analytical techniques for trace element analysis like atomic absorption spectrometry, inductively coupled plasma atomic emission and mass spectrometry, ICP-AES and ICP-MS, respectively, in the last two decades.
The ultimate use of XRF for medical analysis is in vivo measurements made directly in the living patient or volunteer. It started with quantitative analysis of iodine in the human thyroid. The idea sprang from the pioneering work by Jacobsson,1 who developed a technique for subtraction radiology of iodine using two x-ray energies, one above and one below the K-absorption edge of iodine. Hoffer et al.2 realised that if that technology worked, there should be a chance to see the emitted characteristic x-rays from iodine using the semiconductor detectors, which at that time had been developed for nuclear and particle physics. In this way the first in vivo XRF analysis was done, quantifying the iodine concentration in human thyroid, typically around 400 µg g–1.
The further development of the in vivo XRF technique was related to the analysis of heavy elements, first covering lead3–7 and later cadmium8 and to some extent also mercury9 in occupationally exposed workers. Platinum was also analysed to investigate uptake and kinetics of the cytostatic agent cis-platinum in tumour patients.10 The following section describes efforts made to study various toxic elements in vivo in occupationally exposed workers and in patients.
XRF in vivo
Lead
In vivo XRF analysis of lead in human bone has given new information about levels in occupationally exposed smelter workers. Studies clearly show that for workers with an ongoing exposure, blood lead analysis is a poor estimate of the body lead content (over 90% of lead resides in the skeleton). For body burden or integrated exposure measurements, complementary in vivo XRF measurements in bone have to be done. This is a way to identify individuals who carry an internal source of lead, which—when released—may create acute lead intoxication. Such high contamination levels should of course never be accepted in any modern work place.
In vivo XRF analysis has been also shown to be a very important technique in countries like the USA and Canada, where lead intoxication due to lead-based paint still is a very serious problem, especially for children.
Lead in finger bone can be measured by irradiating the finger by two 57Co (122 and 136 keV) sources mounted opposite to each other, see Figure 1. The lead K shell x-rays (K-XRF) and incoherently scattered photons are detected using a high purity germanium detector.
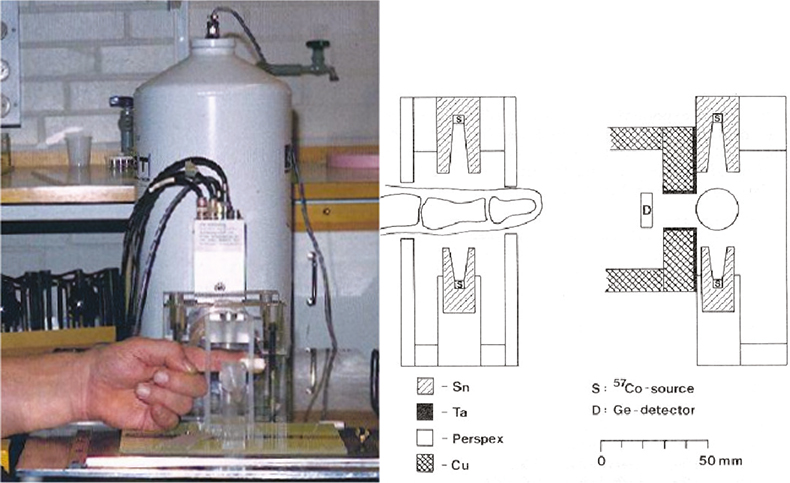
Figure 1. Arrangement for the determination of lead in finger-bone by XRF. Radiation shielding, two 57Co-sources (around 300 MBq each) and the finger-holder are shown.
A different K-XRF bone lead technique, for sampling of lead in tibia, calcaneus and patella, is based on an 88 keV 109Cd source and one large or several small area detectors.11 The technique now seems ready for measurements even on subjects from the general population.
Cadmium
Cadmium has mainly been a problem in the battery industry (Ni–Cd) among welders and in the production and use of paint. Cadmium in tobacco has also been shown to be an important exposure source to smokers. There are also increasing levels of cadmium in society due to releases from industries and waste combustion facilities and from the increased use of fertilisers in agriculture.
For in vivo analysis, XRF is more favourable than the alternative technique using neutron activation, due to a lower radiation exposure and a lower detection limit.
Cadmium in kidneys and liver is readily measured with K-XRF, using polarised photons from a modified x-ray apparatus,8 see Figure 2. Kidney localisation is made using ultrasound. Today measurements can be made, not only in occupationally exposed subjects, but also in the general population. At a group level, smokers can be distinguished from non-smokers by in vivo measured kidney cadmium concentrations.
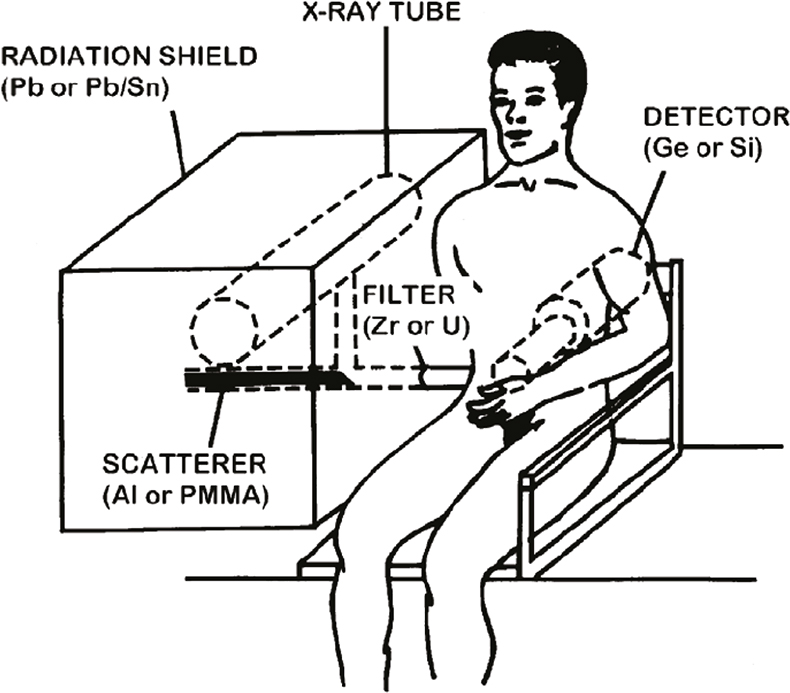
Figure 2. Measurement set-up for in vivo XRF of cadmium (and mercury).15
Mercury
Metallic mercury may be a problem in the electrochemical industry and also in connection with small-scale gold mining, where gold and silver in ore is amalgamated with mercury. Final recovery of fine gold particles extracted is done through heating or burning of the amalgam causing high mercury emissions to the atmosphere and internal and external contamination of people. The main group of humans exposed are gold dealers in shops, rather than the gold miners who work outdoors. In the case of methyl mercury, the populations downstream along the rivers constitute the group at risk, as they depend on fish as their main source of protein. XRF in vivo has the potential to identify the most contaminated and to monitor the influence of improvements in the routines used.
Mercury has been measured in vivo in heavily exposed workers from the thermometer and chlor-alkali industry. Levels above 50 µg g–1 were noted in the kidney and a correlation between urine and kidney mercury was observed.9 The XRF technique used is similar to that used for measurements of cadmium.
Iodine
The thyroid gland is dependent on iodine for production of the thyroid hormones triiodothyronine (T3) and thyroxin (T4), through which the thyroid gland governs our body function and development. In the thyroid, iodine is enriched and stored in the thyroid iodine pool. Knowledge of the iodine pool is most valuable since it can contribute to the understanding of the iodine content in food, thyroidal diseases and possibly predict treatment outcome. This information can be obtained in vivo by XRF.
After the initial work by Hoffer et al.2 and the development of the XRF scanner, many studies on intrathyroidal iodine, using XRF, were performed. After some years, interest in the technique decreased until recently when Hansson12 reinvestigated the technique for analysis of iodine in vivo and for further optimisation. She has also reported very clear differences in iodine concentration in thyroid cancer tissue and in normal thyroid tissue. Moreover, iodine content and distribution in benign and malignant thyroid tissue together with tissue from thyroid healthy subjects was analysed with secondary ion mass spectrometry (SIMS) as a complementary method. The results confirm the significant difference in iodine content between benign and malignant thyroid tissue.
As most x-ray contrast agents contain iodine, contrast molecules can be traced by XRF in vivo, e.g. by measurements in a blood rich finger tip. Alternatively, blood samples have been collected and analysed by XRF. This has opened the possibility to make quantitative determinations of glomerular filtration rate (GFR), either after, for example, a urography or as a separate investigation using small amounts of the contrast agent.13 In a comparison between the x-ray contrast agent Iohexol and the most commonly used marker for clearance measurements, 51Cr-EDTA, there was a strong correlation between the results from the two methods. Iodine is measured using a 241Am (59 keV) source and a silicon detector in a 90° geometry.
Several other elements have been measured in vivo or have shown a potential for such measurements (see Table 1). For more details, the reader is referred to review papers by Börjesson and Mattsson,14 Chettle,7 and Börjesson and Mattsson.15
Table 1. Application, principal measurement site(s), K absorption energy and characteristic x-ray energies of elements subject to in vivo XRF.
Element (Z) | Application | In vivo measurement site(s) | K absorption energy (keV) | Kα x-ray energies (keV) |
Iron (26) | Medical | Eye, skin | 7.11 | 6.39, 6.40 |
Copper (29) | Medical | Eye, skin | 8.98 | 8.03, 8.05 |
Zinc (30) | Medical | Eye, skin | 9.66 | 8.62, 8.64 |
Arsenic (33) | Environmental | Skin | 11.87 | 10.51, 10.54 |
Strontium (38) | Natural | Bone | 16.11 | 14.10, 14.17 |
Silver (47) | Occupational, medical | Skin | 25.51 | 21.99, 22.16 |
Cadmium (48) | Occupational, environmental | Kidneys, liver | 26.71 | 22.98, 23.17 |
Iodine (53) | Natural, medical (X-ray contrast) | Thyroid, blood | 33.17 | 28.32, 28.62 |
Xenon (54) | Medical | Brain | 34.56 | 29.46, 29.78 |
Barium (56) | Medical | Lungs | 37.44 | 31.82, 32.19 |
Platinum (78) | Medical | Kidneys, liver, tumours | 78.40 | 65.12, 66.83 |
Gold (79) | Medical | Kidneys, liver, bone joints | 80.73 | 66.99, 68.81 |
Mercury (80) | Occupational, environmental | Kidneys, liver?, thyroid, bone? | 83.10 | 68.89, 70.82 |
Lead (82) | Occupational, environmental | Bone, kidneys? | 88.00 | 72.80, 74.97 |
Bismuth (83) | Medical | Stomach?, intestines?, brain tumour? | 90.53 | 74.81, 77.11 |
Thorium (90) | Medical | Liver, spleen | 109.65 | 89.96, 93.35 |
Uranium (92) | Nuclear weapon industry, “war”, “crime” | Bone, lung | 115.61 | 94.66, 98.44 |
XRF measurements on samples (XRF in vitro)
In vitro XRF is primarily used for laboratory analysis of, for example, metals, minerals, samples of the environment, food, body fluids and tissue specimens. XRF can also be used to analyse elements of medical interest in blood, urine, sweat, skin, nails, hair, teeth, biopsies of tissues and organs, exhaled air as well as in drinking water and food.
The analysis of a larger number of trace elements in biological samples requires an XRF technique with an ultra high sensitivity and a high spatial resolution. Moreover, a capability for non-destructive, non-vacuum and simultaneous multielemental analyses is needed. Today, it is possible to have a MDC (minimum detectable concentration) of 10–9 (or ng g–1) and x-ray µ-beams with sub-µ-metre size using a combination of focusing mirror and pinhole or capillary technique.
Element concentrations in blood and urine are readily analysed by, for example, chemical techniques (AAS, atomic absorption spectrophotometry, ICP-MS etc.) and atomic/nuclear techniques (XRF, PIXE, particle-induced x-ray emission etc.). A disadvantage of the PIXE method, compared with XRF, is that PIXE may destroy the sample due to very high local energy absorption. However, with proper conditions fulfilled, even µ-PIXE using a scanning proton microprobe, can be used to detect most elements with Z ≥ 10 with detection limits of 1–10 µg g–1.
Total reflection x-ray fluorescence (TXRF) analysis enables us to carry out quantitative analysis of ultra trace elements using trace amount of samples,16 e.g. obtained from a biopsy. Trace element analysis of biopsy samples represents a difficult analytical problem due to the small masses of the samples. Advantages of TXRF analysis over conventional XRF analysis include extremely low background conditions, extremely small amount (volume) of a sample required (a few microlitres) and a multi-elemental analysis technique.
A small number of the existing XRF spectrometers are of the TXRF type and used for analysis of very small samples (≈ µL) and with a current detection limit of attogram (10–18 g). Normal energy dispersive XRF uses larger sample sizes, 1–10 g, and the detection limit is orders of magnitude larger (0.1–10 µg g–1).
The radiation source for in vitro XRF may be a radionuclide, an x-ray tube or an accelerator producing synchrotron radiation (SR). SR-XRF analysis of biological and medical samples will represent an important step in understanding the role of trace elements in biological systems. As sensitivity and spatial resolution improves, new applications open up, as can be seen from the current number of synchrotron radiation-based studies, for which source brightness and the possibility for mapping microscopic distribution of elements are vital features.
The x-ray microbeam technique can be combined with histological examination.17 This technique is useful for toxicology, environmental exposure and industrial health monitoring clinical science. Today there are even focused micro-beams of sub-micrometer size, which allow for determination of the elemental distribution in a single cell. Another application of µ-beam XRF is analysis of metal binding in metalloprotein. As developed recently, gel electrophoresis to clarify changes in the protein structure and SR-XRF analysis for the detection of metal will become a convenient combination method for protein chemistry.
Even for thick target samples, some authors claim that the recent SR facilities with 40–100 keV monochromatic x-rays may offer K-XRF analysis much better than that of an ordinary x-ray tube. However, others found that XRF using an x-ray tube would still be the best way of analysing elements in a thick target sample. Under all circumstances, the availability of synchrotron radiation facilities is very limited.
An extensive review of in vitro techniques and recent developments/applications was presented by Szalóki et al.18 Table 2 lists some examples from which XRF, TXRF and SR-XRF have been used for the analysis of samples from man and his environment.
Table 2. Some examples where XRF, TXRF or SR-XRF have been used for analysis of samples from man and his environment.
Element (Z) | Application | In vitro measurement site |
Calcium (20) | Changes in calcium concentration and distribution | Hair, bone biopsies |
Chromium (24) | Induction of lung cancer due to phagocytosis of lead-chromate particles | Lead-chromate particles in air-ways |
Iron (26) | Elemental composition in breast tissue | Female breast; tumour and healthy tissue |
Nickel (28) | Detection of allergens | Skin |
Copper (29) | Elemental composition in breast tissue | Female breast; tumour and healthy tissue |
Zinc (30) | Elemental composition in breast tissue | Female breast; tumour and healthy tissue |
Bromine (35) | Determination of extra cellular water using stable bromine dilution | Blood samples |
Palladium (46) | Studies of palladium pollution in the environment (car catalyst) | Samples from the environment |
Iodine (53) | Iodine kinetics | Blood and thyroid biopsies |
Platinum (78) | Treatment of cancer patients | Blood, urine, biopsies |
Gold (79) | Treatment of rheumatic illnesses | kidneys, liver, bone joints |
Mercury (80) | Mercury intoxication | Hair, “amalgam tattoos”, samples from dental clinics |
Lead (82) | Lead intoxication; modern and historic perspectives | Paint “flakes”, household ceramics, Lead-chromate particles in air-ways, lead in ancient bones |
Thorium (90) | Risk for cancer induction | Thorotrast |
For more details on in vivo and in vitro XRF in the medicine field, the reader is referred to some recent review papers (Börjesson and Mattsson14,15 and Chettle,7,19) and to the recent issue of the journal X-Ray Spectrometry, Vol. 37, No. 1 (2008).
References
- B. Jacobsson, “Dichromatic absorption radiography, dichromography”, Acta Radiol. 39(6), 437–452 (1953). https://doi.org/10.3109/00016925309136730
- P.B. Hoffer, W.B. Jones, R.B. Crawford, R. Beck and A. Gottschalk, “Fluorescent thyroid scanning: A new method of imaging the thyroid”, Radiology 90, 342–344 (1968). https://doi.org/10.1148/90.2.342
- L. Ahlgren, K. Lidén, S. Mattsson and S. Tejning, “X-ray fluorescence analysis of lead in human skeleton in vivo”, Scand. J. Work Environ. Health 2, 82–86 (1976). https://doi.org/10.5271/sjweh.2815
- L. Ahlgren and S. Mattsson, “An X-ray fluorescence technique for in vivo determination in a bone matrix”, Phys. Med. Biol. 24, 136–145 (1979). https://doi.org/10.1088/0031-9155/24/1/011
- L.J. Somervaille, D.R. Chettle and M.C. Scott, “In vivo measurement of lead in bone using x-ray fluorescence”, Phys. Med. Biol. 30(9), 929–943 (1985). https://doi.org/10.1088/0031-9155/30/9/005
- A.C. Todd and D.R. Chettle, “In vivo X-ray fluorescence of lead in bone: review and current issues”. Environ. Health Perspect. 102(2), 172–177 (1994). https://doi.org/10.1289/ehp.94102172
- D.R. Chettle, “Occupational nuclear medicine: Trace element analysis of living human subjects”, J. Radioanal. Nucl. Chem. 268(3), 653–661 (2006). https://doi.org/10.1007/s10967-006-0206-6
- J.-O. Christoffersson and S. Mattsson, “Polarised X-rays in XRF-analysis for improved in vivo detectability of cadmium in man”, Phys. Med. Biol. 28, 1135–1144 (1983). https://doi.org/10.1088/0031-9155/28/10/005
- J. Börjesson, L. Barregård, G. Sällsten, A. Schütz, R. Jonson, M. Alpsten and S. Mattsson, “In vivo XRF analysis of mercury: the relation between concentrations in the kidney and the urine”, Phys. Med. Biol. 40, 413–426 (1995). https://doi.org/10.1088/0031-9155/40/3/006
- R. Jonson, S. Mattsson and B. Unsgaard, “A method for in vivo analysis of platinum after chemotherapy with cisplatin”, Phys. Med. Biol. 33, 847–857 (1988). https://doi.org/10.1088/0031-9155/33/7/008
- H. Nie, D. Chettle, L. Luo and J. O’Meara, “In vivo investigation of a new 109Cd gamma-ray induced K-XRF bone lead measurement system”, Phys. Med. Biol. 51(2), 351–360 (2006). https://doi.org/10.1088/0031-9155/51/2/011
- M. Hansson, X-ray fluorescence analysis (XRF) for determination of the thyroid iodine content. PhD Thesis, Department of Radiation Physics, Göteborg University, Sahlgrenska University Hospital, Göteborg, Sweden (2008).
- T. Grönberg, S. Sjöberg, T. Almén, K. Golman and S. Mattsson, “Noninvasive estimation of kidney function by x-ray fluorescence analysis. Elimination rate and clearance of contrast media injected for urography in man”, Invest. Radiol. 18, 445–452 (1983). https://doi.org/10.1097/00004424-198309000-00008
- J. Börjesson and S. Mattsson, “New applications. X-ray fluorescence analysis in medical sciences”, in X-ray spectrometry: Recent technological advances, Ed by K. Tsuji, J. Injuk and R. Van Grieken. Wiley, pp. 487–515 (2004). https://doi.org/10.1002/0470020431.ch7
- J. Börjesson and S. Mattsson, “In vivo X-ray fluorescence measurements of lead, cadmium and mercury in occupational and environmental studies: A review of work conducted in Sweden 1970–2005”, X-Ray Spectrom. 37(1), 58–68 (2008). https://doi.org/10.1002/xrs.995
- P. Wobrauschek, “Total reflection x-ray fluorescence analysis—a review”, X-Ray Spectrom. 36(5), 289–300 (2007). https://doi.org/10.1002/xrs.985
- N. Zoeger, C. Streli, P. Wobrauschek, C. Jokubonis, G. Pepponi, P. Roschger, J. Hofstaetter, A. Berzlanovich, D. Wegrzynek, E. Chinea-Cano, A. Markowicz, R. Simon and G. Falkenberg, “Determination of the elemental distribution in human joint bones by SR micro XRF”, X-Ray Spectrom. 37(1), 3–11 (2008). https://doi.org/10.1002/xrs.998
- I.I. Szalóki, S.B. Torok, C.U. Ro, J. Injuk and R.E. Van Grieken, “X-ray spectrometry”, Anal. Chem. 72(12), 211R–233R (2000). https://doi.org/10.1021/a1000018h
- D. Chettle, “X-ray spectroscopy in medicine”, X-Ray Spectrom. 37(1), 1–2 (2008). https://doi.org/10.1002/xrs.1008






![Illustration of a thin film solar cell based on a CIGS [Cu(In,Ga)Se2] absorber layer](/sites/default/files/styles/thumbnail/public/articles/Thin_Film_30-1_F1w.jpg?itok=oNor7cmJ)