New ISO committee for reference materials committed to excellence in accurate measurement results
John P. Hammond
Technical Manager, Starna Scientific Limited, 52–54 Fowler Road, Hainault, Essex IG6 3UT, UK
DOI: https://doi.org/10.1255/sew.2021.a24
© 2021 The Author
Published under a Creative Commons BY-NC-ND licence
Introduction
This article, the fourth in the series, details the ISO technical committee that is responsible for reference materials (RMs) etc.1 This Reference Material committee, formally ISO/REMCO, has now been reorganised by ISO as TC 334. The history of ISO/REMCO is discussed, together with the probable changes in this technical committee brought about by its conversion to TC 334.
There has always been, and will continue to be, collaboration between individual producers of RMs, and in many countries around the World, you will find national “mirror” committees reflecting and inputting regional decisions into their appropriate ISO representatives. However, many years ago, RM producers recognised that the growing need by the analytical community for a number and variety of RMs as well as a need for the assurance of the quality of RMs called for collaboration at the international level. This has been achieved through REMCO, the Council Committee on Reference Materials of the International Organization for Standardization (ISO), which celebrated its 40th anniversary in 2016. The evolution of this organisation and its conversion into the formal ISO TC 334—Reference Material continues in 2021 and beyond, and the key likely changes are detailed here.
1st Generation: the years between 1940 and 1975
The first serious effort towards international cooperation in the field of RMs was the Symposium on an International Reference Materials Program held in May 1969 at the then National Bureau of Standards (NBS), in Washington, DC, under joint sponsorship by the International Committee on Weights and Measures (CIPM) and NBS. It was recognised that the need for RMs was greater than ever before in history and that cooperation on an international scale was needed to meet the World’s future needs. The Symposium recommended that the International Bureau of Weights and Measures (BIPM) be asked to establish an organisational mechanism to gather and disseminate information on the availability of RMs, their characteristics and prices, coordinate information on the needs for standard reference materials (SRMs), identify potential suppliers of RMs, and coordinate information on potential RM certifying facilities. Subsequent to the symposium, CIPM had to decline this role because the limitations of its charter and available resources allowed it to assume only the responsibility for SI base unit metrological SRMs.
Given the lack of progress on this important topic, in November 1973 an ad hoc International Meeting on RMs, under the sponsorship of the International Organization of Legal Metrology (OIML) was held at NBS in Washington, DC. Six international organisations and 12 countries were represented. It was recommended that an independent International Commission on Reference Materials (REMPA) be formed to define and gather and disseminate information on RMs as to their availability, ordering information, properties certified etc. and recommend a plan of action to increase their availability on an international scale.
2nd Generation: the years 1975 to 2000
REMPA met first in April 1975 at which time it established two working groups.
In September 1975, the ISO Council transformed REMPA into the Council Committee on Reference Materials (REMCO) with the following six terms of reference. It is interesting to compare the evolution of these terms with the final versions of ISO/REMCO from 2020 shown in blue.
- To establish definitions, categories, levels and classification of RMs for use by ISO.
To establish concepts, terms and definitions related to RMs. - To determine the structure of related forms of RMs.
To specify the basic characteristics of RMs as required by their intended use. - To formulate criteria to be applied for choice of sources for mention in ISO documents (covering also legal aspects).
To propose actions on RMs required to support other ISO activities. - To prepare guidelines for technical committees for making reference to RMs in ISO documents.
To prepare guidelines for ISO Technical Committees when dealing with reference material issues. - To propose, as far as necessary, action to be taken on RMs required for ISO work.
To communicate with other international organisations on reference material matters. - To deal with matters within its competence arising in relation with other international organizations and to advise Council on action to be taken.
To advise the ISO Technical Management Board (TMB) on reference material issues.
The first meeting of REMCO took place in January 1976, just prior to the International Round Table on Reference Materials.
The mandate of REMCO was to carry out and encourage a broad international effort for the harmonisation, production and application of certified reference materials (CRMs), and during this period REMCO developed a broad series of ISO Guides on RMs:
ISO Guide 6. “Mention of reference materials in International Standards”
This Guide was incorporated into the ISO Directives for technical work as Annex 2c in February 1980 and is, therefore, not recognised today as a separate entity.
ISO Guide 30 “Terms and definitions used in connection with reference materials”: harmonises the vocabulary used in connection with RMs
ISO Guide 31 “Reference materials—Contents of certificates and labels”: ensures that users have sufficient information on a CRM
ISO Guide 32 “Calibration of chemical analysis and uses of certified reference materials” was intended for users of RM in calibration for chemical analysis
This Guide was incorporated into a scheduled revision of ISO Guide 33 in the 3rd Generation.
ISO Guide 33 “Uses of certified reference materials”: describes how to use CRMs in widely diverse fields”
ISO Guide 34 “General requirements for the competence of reference material producers”: was developed to give users increased confidence in the “quality” of CRMs
ISO Guide 35 “Certification of reference materials—General and statistical principles”: was intended mainly for producers of CRMs
At the 14th meeting in May 1989, REMCO decided that the subjects of traceability and levels of RMs were very complicated and reduced its terms of reference to revising ISO Guide 30—“Terms and definitions of reference materials” and harmonising it with the terminology in metrology.
The 15th meeting of REMCO in May 1991 saw Mr S. Rasberry (ANSI, NIST) take the Chair of REMCO. A revised work programme was set out for 1991–1996, and a specific Task Group “Accreditation” was added, tasked as follows:
- To assess the need for the accreditation of RM producers.
- To collect, assess and analyse viewpoints and documentation concerning the accreditation of RM producers.
- To provide liaison with appropriate national and international organisations concerned with accreditation of RM producers.
- To produce a draft ISO guide on the requirements for accrediting RM producers.
- To coordinate future revisions of the resultant ISO guide.
The 17th meeting of REMCO in April 1994 was given strong support for its activities in accreditation by the ISO Central Secretariat. The latter foresaw the need for the international accreditation of RM producers under ISO 9002 or compatible quality system and believed that REMCO had the potential to be recognised as the authoritative body to register RM producers.
The 18th meeting of REMCO in April 1995 led to an initiative to prepare a position paper to examine possible options, including their pros and cons, for establishing some form of international recognition for RM producers.
The 20th meeting of REMCO was strongly focused on draft revised Guide 34 based on the ILAC document. The ILAC representative to REMCO agreed to take the draft revised Guide 34 to ILAC for collaboration to produce a mutually agreed-to document.
The Accreditation Task Group also agreed to produce a draft document on establishing a “Register” of CRM producers that also included “quality” statements about each producer. The intent was to put this Register of CRM producers on the Internet via the ISO server.
REMCO undertook a new work initiative to compile a list of problems faced by CRM producers due to differences in legal and other requirements for labelling, packaging and shipping these materials to different countries in the World. This resulted in the associated Technical Report, ISO/TR 11773:2013—Global distribution of reference materials.
The 21st meeting of REMCO was held in April 1998 and saw the beginning of collaboration with the Pharmacopoeial Discussion Group (PDG). This Group has prepared reference substances for over 70 years and the use of most of these reference substances is mandated by legislation in the member countries. However, these materials do not strictly comply with the VIM definition for “certified reference material”, and to this day the vast majority are certified for specific applications within the pharmacopoeial requirements, and do not contain an associated Expanded Uncertainty budget for the assigned value. In 1998, the PDG wanted to develop a mechanism to have their RMs accepted as “CRMs” since the term “reference materials” gives the perception of lower quality. REMCO members were divided on this issue but agreed to collaborate with PDG to harmonise the concepts and principles related to the certification of RMs. Over 20 years later, this debate still rumbles on; the pros and cons of which will be expanded upon in the next article in this series, when the history/development of the Quality requirements of the whole GxP pharmaceutical environment will be reviewed.
At the 22nd meeting of REMCO in April 1999, REMCO approved the final draft of ISO Guide 34 for submission to the ISO TMB for acceptance by its national member bodies, and also requested its ILAC representative to ask ILAC to accept and to use the revised Guide 34 instead of the corresponding ILAC document.
After its publication in 2000, some national standards bodies selected ISO Guide 34 “General requirements for the competence of reference material producers” as the basis for the accreditation of RM producers instead of the corresponding ILAC Guide 12, and so began the process of converting a “Guide” into a “Standard”, i.e. ISO Guide 34 into ISO 17034, a project previously documented in this publication.2 As we shall see later this conversion did set a precedent, that could be seen as the process that initiated the formation of TC 334, but more of that later.
It was during this period (in 1995) that, from a personal perspective, I was invited by Dr Michael Parkany from ISO to present a paper at an International symposium in Melbourne on “Quality Assurance and TQM for Analytical laboratories”.3 It was also at this symposium that I met key personal active in REMCO, and as a consequence was invited on to the Cooperation on International Traceability in Analytical Chemistry (CITAC), and ultimately on to the REMCO committee as a UK Industrial representative, in a role that continues to this day.
3rd Generation: the years 2000 to 2020
The 23rd meeting of REMCO was held in May 2000, and a completely new structure, which was essentially maintained until its disbandment in 2021, was approved. The “Executive Committee” was renamed the “Chairman’s Advisory Group” and was composed of the ISO Secretary, the Chair and Chair-elect and the Convenors of the Task Groups, which were renamed Steering Groups.
During this period, this structure was maintained and adjusted to reflect the current work programme, but the format shown in a graphical format in Figure 1, provides a valuable reference for the 4th Generation discussions detailed below.
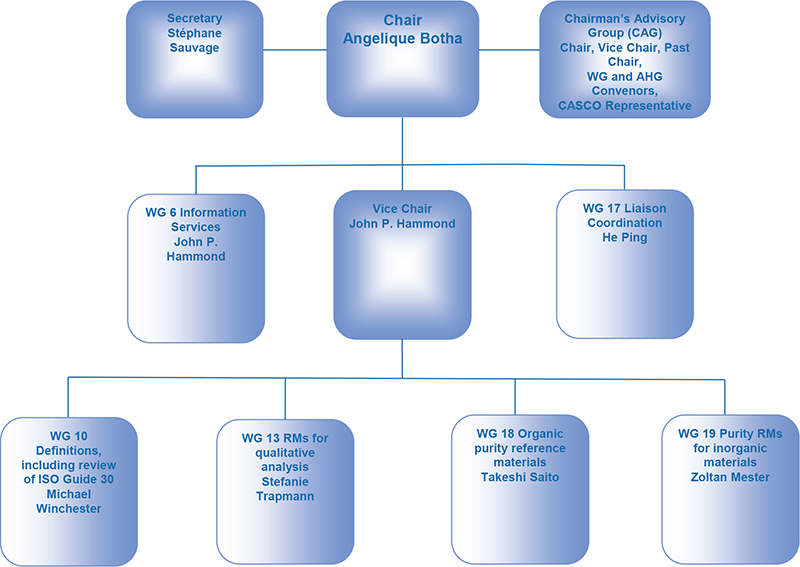
Figure 1. ISO/REMCO Committee structure (2020).
During this period the committee continued to produce both new and revised guidance documents as follows:
- ISO Guide 30:2015 Reference materials—Selected terms and definitions
- ISO Guide 31:2015 Reference materials—Contents of certificates, labels and accompanying documentation
- ISO Guide 33:2015 Reference materials—Good practice in using reference materials
- ISO Guide 35:2017 Reference materials—Guidance for characterization and assessment of homogeneity and stability
- ISO Guide 80:2014 Guidance for the in-house preparation of quality control materials (QCMs)
Guide(s) under development
- ISO Guide 85:202x RMs for qualitative property values
- ISO Guide 86:202x Purity RMs for small organic molecules
- ISO Guide 87:202x Purity RMs for metals and metalloids
- ISO/TR 79:2015 Reference materials—Examples of reference materials for qualitative properties
- ISO/TR 10989:2009 Reference materials—Guidance on, and keywords used for RM categorization
- ISO/TR 11773:2013 Global distribution of reference materials
- ISO/TR 16476:2016 Reference materials—Establishing and expressing metrological traceability of quantity values assigned to reference materials
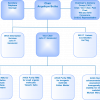
The interrelationship of these documents is shown graphically in Figure 2, in which one should note the following structural aspects:
- At the centre is the standard for Reference Material producers, namely ISO 17034.4
- The adjacent ring provides the associated Guide documents, which are either normative references to ISO 17034, or at the very least should be used for additional guidance associated with ISO 17034.
- The outer ring details other published/pending documents from the TC.
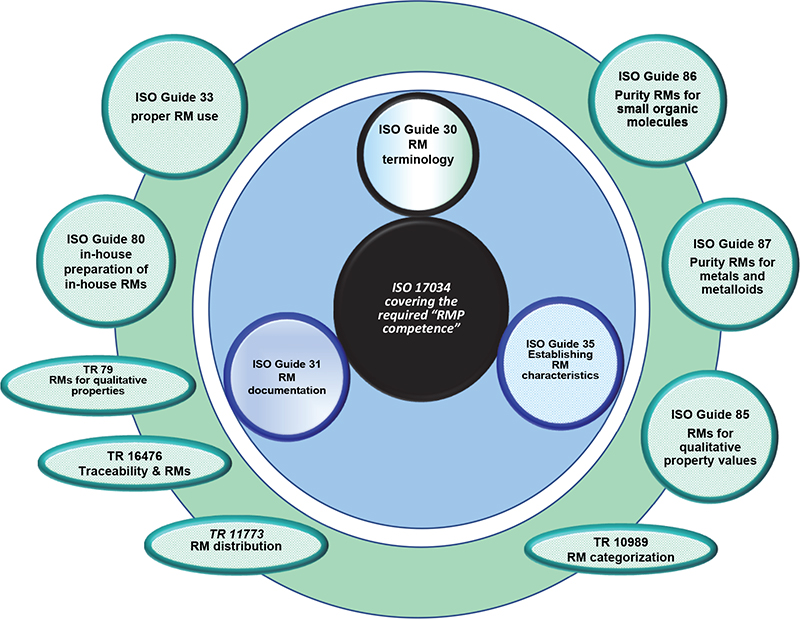
Figure 2. ISO/REMCO Guides and TRs (2020).
4th Generation: from 2021 forward
This essential role of supporting ISO 17034 continues as the primary mandate of TC 334, but here is the first significant change in this TC.
By definition, an ISO Technical Committee’s main role is to write standards, appropriate to its defined area of expertise.
Also, in the language of ISO, a “Guide”, is defined as an informative document which assists an ISO TC in the construction of standards, appropriate to the TC.
However, as you can see from the above list the ISO/REMCO Guides, in some cases, are not designed for this purpose, but are informative documents for RM producers per se. So, the first task that the new TC 334 has to consider is how to meet this requirement and convert these Guides into the appropriate standards.
In addition, in a TC structure a Working Group is tasked with a specific project plan, and associated timeframe to investigate (and produce if appropriate) a related standard; and as one can see from the final ISO/REMCO structure there are a couple of currently defined Working Groups (WG6 and WG17) that do not meet this requirement. Therefore, a structural rearrangement with respect to these essential requirements will be required.
These changes, together with other inter-related communication/liaison requirements will be discussed at the upcoming inaugural virtual TC 334 meeting in early September 2021; and we will keep you updated in the Quality Matters column, as these details are released.
So, on reflection, and given the historical evolution of “Guides” into “Standards”, c.f. ISO Guides 25, and 34 into ISO 170255 and ISO 17034,4 respectively, ISO/REMCO to ISO TC/334 is not really an unexpected transition, is it?
The next (and future) article(s) will discuss similar and evolving Quality environments.
“Will their paths converge… only time will tell?”
References
- ISO Press Release, https://www.spectroscopyeurope.com/news/new-iso-committee-reference-materials
- J. Hammond, “Into the future (Part 2): changes to ISO 17025 and ISO Guide 34”, Spectrosc. Europe 29(4), 11 (2017). https://www.spectroscopyeurope.com/quality/future-part-2-changes-iso-17025-and-iso-guide-34
- M. Parkany (Ed.), Quality Assurance and TQM for Analytical Laboratories. The Royal Society of Chemistry, Special Publication No. 169 (1995). ISBN 0-85404-760-3
- ISO 17034, General Requirements for the Competence of Reference Material Producers. International Organization for Standardization (ISO), Geneva, Switzerland (2016).
- ISO/IEC 17025, General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO), Geneva, Switzerland (2017).

John Hammond
John Hammond is an experienced analytical scientist, spectroscopist and technical marketing professional, skilled in the development, production and marketing of analytical systems into highly regulated and controlled industries. A Fellow of the Royal Society of Chemistry (FRSC), executive member of ISO/TC334 and an Expert Advisor to the United States Pharmacopeia, General Chapters, Chemical Analysis committee.
[email protected]