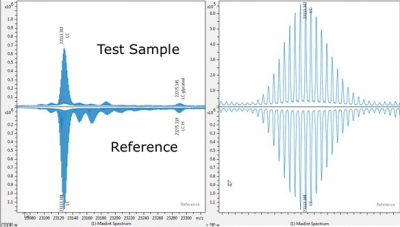
Bruker’s mass spectrometry BioPharma Compass 3.0 solution now includes clone selection and MALDI release identity testing to enhance biopharmaceutical characterisation. It supports both high-resolution ESI and MALDI MS, supporting 21 CFR Part 11 compliance. New butterfly plots allow for sub-unit and intact mass analysis for batch-to-batch comparisons, or originator vs biosimilars analysis. Regulated rapid release identity testing employs MALDI to support “pack and fill” QC. Clone screening using intact Fc/2 glycan profiles is performed by MALDI in applications where speed is beneficial.







