Clemens Anklin
Vice President NMR Applications & Training, Bruker BioSpin
Technology advances that have driven our “always on” culture have led to huge worldwide demand for powerful and, critically, rechargeable batteries. The shift brought about by portable devices such as laptops and mobile phones is the most obvious reason. Add to this the growing popularity of electric cars, increased recognition of climate change and the need for alternative energy solutions and it is clear where the growth trajectory is heading.
This global surge in portable electronics and our insatiable appetite for improved performance is driving research into battery optimisation. The high energy density and electrochemical potential of lithium (Li) has made lithium ion batteries (LIBs) one of the world’s most popular options. Since their initial development in the 1970s, LIBs have enabled significant technological innovation, with the first rechargeable model launched in 1991 by Sony Corporation.
Optimising battery materials and improving transport properties of target ions, all while lowering costs, requires an understanding of the underlying chemistry of their materials. Developments in in situ measurement techniques such as magnetic resonance spectroscopy, including nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR) spectroscopies, and imaging techniques such as magnetic resonance imaging (MRI) are paving the way for progress.
How do lithium ion batteries work?
Rechargeable batteries depend on electrochemical reactions, where chemical energy is converted to electrical energy, and vice versa, via the movement of ions and electrons in an electrolyte between two electrodes, the anode and the cathode.
During discharge, Li-ions carry the current within the battery from the anode to the cathode through the electrolyte and separator. When charging, an external electrical power source applies a higher voltage than the battery produces, forcing a charging current to flow within the battery from the cathode to the anode. The Li-ions then move from the cathode to the anode, where they become intercalated in the porous anode material (Figure 1).
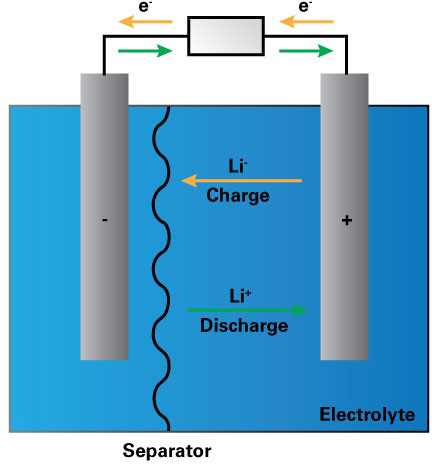
Figure 1. Schematic of how a lithium ion battery works.1
Extending battery life
LIB research is focused on the solid–electrolyte interphase (SEI) formed on the anode during the battery’s first charge, which is crucial to its long-term operation. The formation of a stable SEI determines many performance parameters. During charging, however, when Li ions move towards the anode, they may undergo plating, leading to the formation of dendrites, which can cause the battery to short-circuit and catch fire. Little is known currently about how to prevent dendrite formation.
Understanding the microstructural characteristics of dendrites, growth mechanisms and the role of key factors such as current density, electrolyte salt, solvents and additives is crucial to the progress of LIB research and to ensure battery safety. There are a number of additives used in electrolytes to suppress dendrite formation, but their consumption by the SEI2 often renders them ineffective in the long term.
NMR spectroscopy
Developments in NMR have improved the understanding of SEI by enabling the separation and quantitative identification of many aspects of the layer. For example, 7Li and 19F magic angle spinning (MAS) NMR spectroscopy has allowed the identification and quantification of lithium fluoride (LiF) in the SEI at anodes and electrodes in rechargeable LIBs.3 Measuring the percentage Li loss from the cathode can enable researchers to better understand SEI stability and its impact on LIB life.
Dynamic nuclear polarisation (DNP) has been investigated with low temperature (LT) solid-state NMR, enabling surface sensitive characterisation of the complex, heterogeneous SEI layer. Leskes et al. found that MAS-DNP could increase the sensitivity of SEI detection on reduced graphene oxide (rGO) anodes in a Li-ion cell.4 Natural abundance 13C spectra could be obtained in a matter of hours (Figure 2), in contrast to NMR without DNP which requires isotope enrichment and takes more than a day to acquire signals. This could provide fingerprints of the organic phases comprising the SEI. This approach can be used to study novel electrolyte compositions and develop electrolyte additives that can be used to tailor and control the nature of the SEI layer.
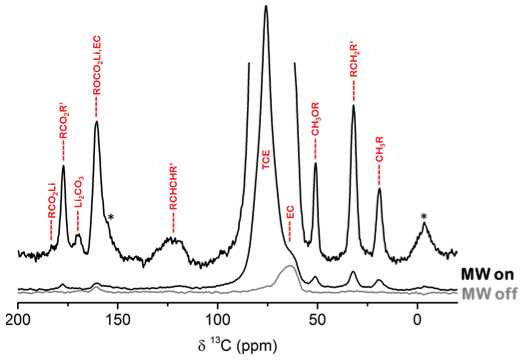
Figure 2. 1H-13C CP MAS-DNP spectrum of a cycled rGO electrode that was impregnated with 16 mM TEKPoL in TCE acquired at 100 K and 10 kHz MAS without (grey) and with (black) microwave irradiation. Sidebands are labelled with asterisk and possible assignment of functional groups/phases in the SEI is shown in red. Adapted with permission from Leskes et al., “Surface-sensitive NMR detection of the solid electrolyte interphase layer on reduced graphene oxide”, J. Phys. Chem. Lett. 8, 1078–1085 (2017). https://doi.org/10.1021/acs.jpclett.6b02590. Copyright 2017 American Chemical Society.
Dendritic growth can also be monitored and quantified using NMR methods. Changes in the intensity of the Li peak during cycling can be correlated with the growth of dendritic microstructures vs smoothly deposited metal. One study found that in situ NMR could determine that up to 90 % of Li deposited during slow charge of a Li/LiCoO2 battery was dendritic.5 NMR can be used to systematically test methods of dendrite suppression, such as electrolyte additives, advanced separators, cell pressure, temperature and electrochemical cycling conditions.6 This, together with the quantitative measurement of SEI and novel battery materials in operando, shows that NMR spectroscopy can help in the development of innovative LIB design.
EPR: a complementary technique
In addition to NMR, EPR spectroscopy is well suited to studying the evolution of metallic Li species in operando. Compared with NMR, EPR has a higher surface selectivity because of the low penetration depth of microwaves into the bulk, enabling differentiation between bulk and fine structured lithium dendrites (Figure 3).7
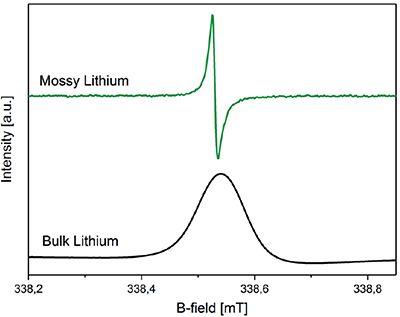
Figure 3. Different lithium morphologies detected with a Bruker E540 ELEXSYS X-band spectrometer equipped with a 4108 TMHS resonator. Top: mossy lithium (green); bottom: bulk lithium (black). Adapted from Reference 7 in accordance with Creative Commons Attribution 4.0 International License.
EPR imaging is now being used to investigate the formation and disappearance of radical oxygen species in new batteries as a function of current rates, potentials, resting times, electrolytes or temperatures.
Gaining spatial information with MRI
In addition to spectroscopy, MRI is a powerful, non-invasive technique to provide time-resolved and quantitative information about the changes occurring within the electrolyte and electrodes of a LIB. Similar to NMR, MRI is capable of detecting and localising lithium microstructure build-up, but has the additional benefit of providing spatial information, allowing specific structural changes to be localised. Researchers have been able to reconstruct 3D images of growing Li dendrites, elucidating their growth rate and fractal behaviour (Figure 4).8
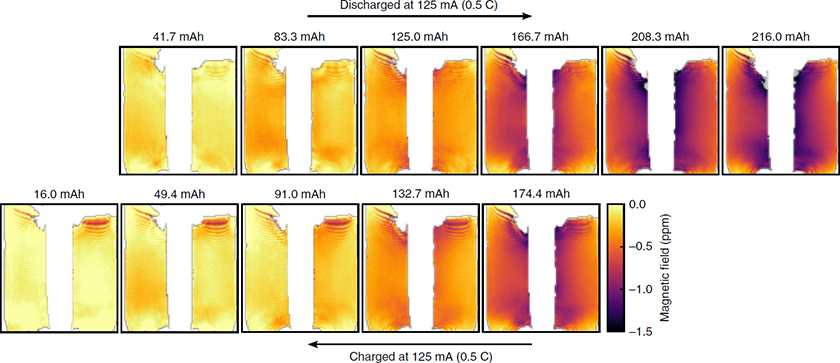
Figure 4. Series of magnetic field maps taken at intervals during discharge and then charge of the cell. The plots are labelled by the discharge capacity of the cell at each step. The magnetic field maps are referenced to the field map produced by the fully charged cell. Reproduced from Reference 9 in accordance with Creative Commons Attribution 4.0 International License.
The battery of the future
Developments in analytical technologies over the past 40 years have significantly impacted the battery industry. Where techniques such as electron and optical microscopy offer high resolution imaging, they are often limited to surface imaging and are difficult to interpret quantitatively. NMR and EPR spectroscopy are both non-invasive methods with quantitative capabilities, and research is continuing to improve their sensitivity and increase resolution.
Significant advances have been made in recent years to improve the capabilities of rechargeable LIBs. A deeper understanding of possible alternative electrode materials, electrolyte components (Li salts, solvents and additives), and the processes governing SEI and dendrite formation, is paving the way for safer LIBs with higher energy densities.
The rapid development of new materials, such as higher capacity cathodes with higher operating voltage, can pose challenges for electrolyte and interphase chemistry. Innovations are being met with sophisticated analytical technology, such as EPR and NMR spectroscopy and MRI, to ensure that LIB research continues to deliver energy storage solutions of the future.
References
- Battery Research: Characterizing the Future. Essential Knowledge Briefings, Wiley, Chichester (2019). https://www.essentialknowledgebriefings.com/downloads/battery-research-characterizing-the-future/
- Y. Takeda, O. Yamamoto and N. Imanishi, “Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes”, Electrochemistry 84(4), 210–218 (2015). https://doi.org/10.5796/electrochemistry.84.210
- B.M. Meyer, N. Leifer, S. Sakamoto, S.G. Greenbaum and C.P. Grey, “High field multinuclear NMR investigation of the SEI layer in lithium rechargeable batteries”, Electrochem. Solid-State Lett. 8(3), A145–A148 (2005). https://doi.org/10.1149/1.1854117
- M. Leskes, G. Kim, T. Liu, A.L. Michan, F. Aussenac, P. Dorffer, S. Paul and C.P. Grey, “Surface-sensitive NMR detection of the solid electrolyte interphase layer on reduced graphene oxide”, J. Phys. Chem. Lett. 8, 1078–1085 (2017). https://doi.org/10.1021/acs.jpclett.6b02590
- R. Bhattacharyya, B. Key, H. Chen, A.S. Best, A.F. Hollenkamp and C.P. Grey, “In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries”, Nat. Methods 9, 504–510 (2010). https://doi.org/10.1038/nmat2764
- O. Pecher, J. Carretero-Gonzáelz, K.J. Griffith and C.P. Grey, “Materials’ methods: NMR in battery research”, Chem. Mater. 29, 213–242 (2016). https://doi.org/10.1021/acs.chemmater.6b03183
- A. Niemöller, P. Jakes, R.A. Eichel and J. Granwehr, “EPR imaging of metallic lithium and its application to dendrite localisation in battery separators”, Sci. Rep. 8, 14331 (2018). https://doi.org/10.1038/s41598-018-32112-y
- A.J. Ilott, M. Mohammadi, H.J. Chang, C.P. Grey and A. Jerschow, “Real-time 3D imaging of microstructure growth in battery cells using indirect MRI”, Proc. Natl. Acad. Sci. 113(39), 10779–10784 (2016). https://doi.org/10.1073/pnas.1607903113
- A.J. Ilott, M. Mohammadi, C.M. Schauerman, M.J. Ganter and A. Jerschow, “Rechargeable lithium-ion cell state of charge and defect detection by in-situ inside-out magnetic resonance imaging”, Nat. Commun. 9, 1776 (2018). https://doi.org/10.1038/s41467-018-04192-x