O. Samek,a P. Zemánek,a S. Bernatová,a Z. Piláta and H.H. Telleb
aInstitute of Scientific Instruments of the AS CR, v.v.i., Academy of Sciences of the Czech Republic, Kralovopolska 147, 61264 Brno, Czech Republic
bDepartment of Physics, College of Science, Swansea University, Singleton Park, Swansea, SA2 8PP, UK
Introduction
In marine and freshwater systems, thousands of organisms interact with a highly dynamic aquatic environment. Phytoplankton (tiny microscopic plants—algae), serve as the base of the aquatic food web, because of their ability to synthesise polyunsaturated fatty acids. Phytoplankton are then consumed by zooplankton which are subsequently consumed by fish. The last members of this food chain are humans eating fish.
It is well known that fish constitute a major source of highly unsaturated fatty acids (HUFA). Thus, choosing fish as part of a person’s diet should be natural in order to satisfy nutritional requirements for both n–3 (Omega-3) and n–6 (Omega-6) HUFA. These are a sub-set of polyunsaturated fatty acids (PUFA) with ≥20 carbon atoms and ≥3 carbon double-bonds (C=C), respectively.1 The selection of food is mostly based on proximity, convenience, taste/odour and ingestibility rather than on specific nutrional requirements. Consequently, humans living in inland areas, where it is difficult and expensive to buy fresh seawater fish, might be affected by not rating fish’s beneficial fatty acids as overly important. At present, most fish consumed inland come from fish farms. In this aquaculture industry, Chile and Norway are the two main global suppliers, who basically control farmed salmon products.
As the number of fish farms grow, it becomes evermore important to guarantee that the high content of precious omega-3 and omega-6 fatty acids find their way into fish oils. This, therefore, dictates some fish dietary requirements while at the same time ensuring optimum, economically viable growth speed of the fish in their aquacultural environment. At present, aquaculture is one of the fastest-growing food production sectors in the world, expanding at 7.5% year-on-year since 1994.2 One specific breakthrough that could help fish farmers is that it has become possible to use algae from which one can extract the desired omega-3 fatty acids, replacing undesirable fish content in fish feed. Eventually, fish farmers might be able to eliminate soy, chicken oil and other terrestrial products entirely; instead, they should feed the fish on plankton, which is easy to grow.
It therefore seems obvious that fast and accurate analytical methods are urgently needed which can be used to estimate whether useful fatty acids are present in fish food and, if that were the case, could also be used to determine if their consumption results in heightened fish lipid content.
It is worth noting that, besides the use of algae in the food chain, more recently attention has turned to the potential exploitation of algae (or specifically algae lipids) for the production of high-octane biofuels.3 This alternative approach, while still very much in its infancy, is gaining credibility; large, established multi-national corporations and universities have announced major research projects to embark on proof-of-principle or even large-scale testing of algae cultivation for mass generation of biofuels.4
Analysis methodologies
Following the two aforementioned challenges—utilisation of algae in aquaculture and the biofuel industry—a fast method for the identification and analysis of lipids/oil should be able to estimate their so-called iodine value (IV). The iodine value reflects the degree of unsaturation of a fatty acid (or oil) and helps to estimate how many carbon–carbon double bonds (C=C) are present in the lipid sample. Therefore, the IV parameter constitutes part of the current biofuel and food industry standards. A high IV suggests a high content of highly unsaturated fatty acids, such as, for example, Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA). Note that both are deemed to be excellent sources for the food industry; however, they are not suitable for biofuel production because they cause undesirable oxidation of biofuel.
Traditionally, IV determination is made by a specific chemical analysis method in which the carbon double-bond reactivity to added iodine is measured.5 The IV is then expressed as grams of iodine taken up per 100 g of sample. However, while standard, this analytical technique involves several time-consuming steps. Thus, a simpler and speedier analytical technique would be desirable, with potential benefits to control/process applications in the food industry (but equally in biofuel applications in the search for the best “fuel-making”algae).
Here, we report on the development of a methodology based on Raman spectroscopy for fast and accurate IV estimation. Briefly, the IV is readily calculated from intensity ratios of specific, selected Raman bands. Comparative evaluation of spectra from all contributors in the food chain will help in understanding lipid profiles in fish feed/fish species. Since no major sample preparation is required, this analysis can be done in situ (for example in pond-production systems) to establish whether the fish have access to an abundant plankton/algae population containing the desired fatty acid mixtures. Consequently, by sampling the fish themselves when caught for food processing, the influence of lipid content in fish feed on the quality of fish oil could be followed and monitored in (nearly) real-time.
Instrumentation
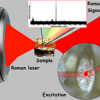
Raman micro-spectroscopic experiments with living algal cells were carried out using an in-house-built experimental system, incorporating a custom-designed inverted microscope frame. Briefly, the Raman laser excitation light (from a Coherent 899-01 Ti:Sapphire laser, operating at l = 785 nm) was delivered to the set-up via an optical fibre. The maximum laser power available for excitation, at the location of the specimen, was measured to be approximately 100 mW. The laser beam was focused onto the specimen with an optimised water-immersion objective lens (Olympus UPLSAPO 60, NA = 1.2). The Raman scattered light was collected through the same objective lens, dispersed with a 600 grooves/mm diffraction grating, imaged onto the chip of a high-sensitivity, liquid-nitrogen-cooled spectroscopic CCD camera (Princeton Instruments Spec-10:100BR/LN) and recorded using the camera’s control software (Princeton Instruments WinSpec). The schematic layout of this system is shown in Figure 1; for further details see References 4 and 6.

Despite using a near infrared laser for Raman scattering excitation and focusing the laser beam directly into the lipid storage bodies, the collected Raman spectra still exhibit noticeable non-specific fluorescence background. Thus, we adopted the rolling circle filter (RCF) technique for background removal.4,7 This post-acquisition processing of the spectra was done off-line, using custom-written routines incorporated into Matlab software (MathWorks).
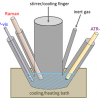
It should be noted that the results discussed below were recorded for in vitro or fixated (algae) samples. For in vivo measurements in a natural environment, algae were trapped by using Raman tweezers; for the latter a secondary trapping laser was used.
Applications
In our experiments, we determined the ratio of unsaturated-to-saturated carbon–carbon bonds in fish and algal lipid molecules and translated these into IV values. In this analysis approach, two characteristic Raman spectral peaks were used,4 namely: the feature at 1656 cm–1, corresponding to the cis-C=C stretching-mode, which is proportional to the amount of unsaturated C=C bonds; the feature at 1445 cm–1 is associated with the CH2 scissoring mode, the intensity of which is proportional to the amount of saturated C–C bonds.
Both lipid-related peaks are highlighted in the spectra panels of Figure 2 for various fish and algal specimens. From the intensity ratio of the two features, I1656 / I1445, the average ratio of double-to-single carbon–carbon bonds n(C=C)/n(CH2) in the specimen can be estimated. This is accomplished by constructing a calibration curve that relates I1656 / I1445 to n(C=C)/n(CH2) and hence IV values, using published IV data of the hydrocarbon chain unsaturation for various pure fatty acids. With such a calibration curve, one is now able to directly convert the measured Raman peak intensities and n(C=C)/n(CH2) ratios to the iodine values, for any given sample (see the main panel in Figure 2); for details of the procedure, see Reference 6. The results of IV estimates for two fish species and two algal samples are included in the plot. From the calibration/measurement curve, one may come to the following conclusions.

Gilt-head bream—both commercially fished and raised in fish farms—may be a good dietry choice of a food with high Omega-3 and Omega-6 content, with an IV ≈ 130, which implies high amounts of Omega-3 fatty acids representing both EPA and DHA in its fish oil. Gilt-head bream is widely available in restaurants serving fish dishes; it should be noted that bream is frequently preferred over salmon (with IV ≈ 180), with the latter perceived as a bit fatty on occasion.
The second fish included in Figure 2 is carp; this is a freshwater fish (domesticated and reared at a pond-production system as food fish, mainly in the Czech Republic). It exhibits an IV ≈ 75, which means a lesser amount of Omega-3 and Omega-6 fatty acids. Nevertheless, from a nutritional point of view, carp may still be seen as being an excellent choice for the uptake of beneficial Omega-3 and Omega-6 fatty acids.
It is clear that, using the above (fast) measurement and evaluation method, one can easily follow the influence of lipid-content in fish feed (either algae eaten by the fish directly in the pond or fish feed prepared for the aquaculture industry) has on the quality of fish oil. Thus, one can monitor the fish’s nutritional value for humans and optimise the best composition of feeding ingredients.
For comparison to the actual fish measurements, Raman spectroscopy experiments for two selected algae species are included in Figure 2. The first is Trachydiscus minutus, for which a quite high IV ≈ 245 is found; this suggests a rather high amount of Omega-3 and Omega-6 fatty acids. Indeed, Trachydiscus minutus is known to have the potential to contain as much as 30% of EPA in its lipid bodies.6 Thus, it might excel in the food industry, namely in fish feed and in algae-derived EPA and DHA formulae (for example, for enhancement of infant formulae). The second species, Botryococcus braunii, has one of the lowest IV values of algae. While just acceptable for fish feed, it may be seen as the species of choice in the biofuel production for which a low IV value is actually required (for biofuel production IV must be kept below IV < 120 because of possible problems with fuel oxidation at high IV).
Conclusions
We have applied Raman spectroscopy to fish/algal lipid research and, in particular, its use for the determination of the iodine value in samples; the IV parameter is seen as one of the important measures of fatty acids composition (it is associated with the unsaturation of fatty acids) and is frequently used in the food and biofuel industries.
The technique of Raman spectroscopy is an excellent candidate to follow the food chain in the aquaculture industry, enabling one to monitor the iodine value of algal-derived fish feed for its lipid content and, thus, revealing possible transgression of feed lipids to fish lipids. Based on this, improvement of the lipid composition in cultured fish (for example from traditional pond production) it should be feasible by changing—normally meaning to increase—the actual lipid content of the feed. Similarly, the same procedure of IV determination can be applied to monitor algae samples for biofuel production, where iodine values must be kept below a given limit, rather than maximising it. We have specifically shown that various fish oils exhibit significantly different iodine values, which may have important implications for the food industry.
It should be noted that Raman spectroscopy can be combined with optical tweezers and with micro-fluidic chips, so that such a hybrid instrument should make it possible to measure lipid content, nutrient dynamics and metabolism in vivo, in real-time and label-free. Thus, with this type of bio-sensing, one may easily detect population variability, which is important for the preparation of fish feed and in biofuel production control. Notably, it is now feasible to employ Raman spectroscopy-based sensors to sort algal cells according to the degree of unsaturation (IV) in lipid storage bodies in-line and in real-time.
We believe that Raman spectroscopy—being a reliable, label-free and non-invasive technique—will be of significant relevance to research groups currently involved in, or intending to join, the quest for sustainable algal feed applications in the aquaculture industry, ranging from biotechnology, over complex interactions in biological systems and genetic engineering, to pharmaceutical and food industry uses and not forgetting biofuel generation.
Acknowledgements
The authors acknowledge the support for O. Samek with a Marie Curie Re-integration Grant (PERG 06-GA-2009-256526). This work also received support from the Ministry of Education, Youth and Sports of the Czech Republic, together with the European Commission and the Czech Science Foundation (ALISI No. CZ.1.05/2.1.00/01.0017 and GA P205/11/1687). The authors would like to thank S. Jonáš for help in setting up the experimental apparatus.
References
- M.T. Arts and C.C. Kohler, “Health and condition in fish: the influence of lipids in membrane competency and immune response”, in Lipids in Aquatic Ecosystems, Ed by M.T. Arts, M.T. Brett and M. Kainz. Springer Science, Heidelberg, Germany, pp. 237–256 (2009).
- S. Simpson, “The blue food revolution: making aquaculture a sustainable food source”, Sci. Am. 305(2), 34–41 (2011).
- Y. Chisti, “Biodiesel from microalgae beats bioethanol”, Trends Biotechnol. 26, 126–131 (2008). doi: 10.1016/j.tibtech.2007.12.002
- O. Samek, P. Zemánek and H.H. Telle, “Characterization of oil-producing microalgae using Raman spectroscopy”, Laser Phys. Lett. 8, 701–709 (2011). doi: 10.1002/lapl.201110060
- G. Knothe, “Structure indices in FA chemistry: how relevant is the iodine value”, J. Am. Oil Chem. Soc. 79, 847–854 (2002).
- O. Samek, A. Jonáš, Z. Pilát, P. Zemánek, L. Nedbal, J. Tríska, P. Kotas and M. Trtílek, “Raman microspectroscopy of individual algal cells: sensing unsaturation of storage lipids in vivo”, Sensors 10, 8635–8651 (2010). doi: 10.3390/s100908635
- N.N. Brandt, O.O. Brovko, A.Y. Chikishev and O.D. Paraschuk, “Optimization of the Rolling-Circle Filter for Raman background subtraction”, Appl. Spectrosc. 60, 288–293 (2006). doi: 10.1366/000370206776342553